Ahmad A. Tarhini, Adjuvant therapy in melanoma, Abstract 9500
Interferon is not a options anymore!
What drugs are the best?
What endpoints we will use: present endpoint is RFS; needs to be changed for OS
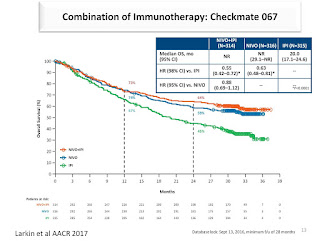
Ipilimumab 10 mg /kg showed benefit for high-risk melanoma patients in the
previous research
In the present study,
abstract 9500: lower dose gives financial advantages but most important less side effects grade III and IV;
New: 10 mg is similar with 3 mg/kg in terms of survival ! (RFS- relapse-free survival)
The question: is this the same for OS (overall survival)?
In metastatic melanoma OS 10 mg showed benefit- thus researchers suspect the same will be seen in the adjuvant setting; conclusion: we have to wait for data on OS in order to decide the dose..
Ipi after Pembro Keynote 006
Caroline Robert,
Abstract 9504
Pembro arms -
What is happening with pts that stop -not because of toxicity but simple because they had benefit;
median follow-up-
104 patients in total
64 ongoing
10 SD (stable disease)
not only the Complete Responses are lasting but also Partial Responses
-2 years was administrated
-efficacy
-safety
Patients received previous immuno in both
Patients were fallowed 9,7 months after completing the treatment with pembro.
''Responses were durable in pts who completed pembro; 9.7 months after completion of pembro, estimated PFS was 91% in all 104 pts, 95% in pts with complete response and 91% in pts with partial response and 83% in pts with stable disease.''
Pembro -duration of response higher.
Jeff Weber (for Georgina Long) Abstract 9505
Dabra/Mek- superior to monotherapy is known
Long term OS in a subset of pts -phase II study
3 arms -the second with lower dose of trametinib (1 mg)
5 years follow up
Patients- treatment naive, BM stable (a subset), LDH was above normal
half pts > 3 metastases
-subsequent therapy-anti PD1 or ipi
RR combi- 76%
RR dabra 54%
OS- 28% at 5 years for all
OS -45% at 5 years for pts with LDH normal
LDH normal - median survival of 4 years.
the main issues:
-do we have long term survivors or cured patients?
-can the treatment be stopped
-who are the ones surviving?
I. long term follow up trial ipi after pembro
keynote 006-most mature data
3 years 50% pembro -with the tendency to form a plateau
primary resistance and secondary
71% DoR
Pembro- is not depending of dose like ipi
Treatments duration of 2 years is not based of scientific data-just clinical observations- a trial is needed to see the optimal duration? (C.Robert)
Characterization of the complete respondents- a must!
Who are the survivors - in general the ones with LDH normal, less disease sites, performance status 0 and CR (complete response); we need for better biomarkers.
Can we stop?- with immuno is clear that after some time (2 years ) could be stopped, with Braf/Mek not known!
Re-challenge- again differences - with immuno there is not enough data showing that can work in case of re-initiation, but with combi Braf/Meks there is evidence re-challenge is working.
Combi MB (Brain Metastasis), Abstract 9506
A. Davies
Break -MB -4 groups of patients A, B, C, D
A- not simptomatic
B -simptomatic
C -ECOG 0-1
D 0-2
intracranial response -primary endpoint 58% in Cohort A
median duration of reponse 6,5 months obs shorter then for extracranial disease (12-14 months)
See the
article in The Lancet.
Check Mate 204- faze II study MB (for patients with brain metastasis), Abstract 9507
Nivo+ipi
No previous treatment!
primary end points -intracranial response
secondary- OS, PFS etc
109 treated
70 patients followed
efficacy Nivo/Ipi
53% global response (intra+extracranial)
67%PFS -at 6 months
time to response of 2,8 months
23 pts stopped because of toxicity
Georgina Long - Trail ABC (BM), Abstract 9508
76 pts
18 months follow up
most patients were male,
younger in cohort A, Braf mutated
previous combi Braf/Mek was permited
Best intracranial response-
A- 42% ipi/nivo; 6 months PFS 46%
B- 20 % nivo alone
C- 6 % leptomeningeal on nivo
46% PFS -6 months
16% response with prior therapy Braf/Mek
Some patients could respond in the brain , but not extra-cranial
Bellow most relevant data from the Brain Metastasis studies -
best results are obtained on asymptomatic patients and the response of patients with leptomeningeal disease remains poor. Clinical trials for the patients with symptomatic disease are considered.
Adjuvante fotemustine versus surveillance in high risk uveal melanoma- no benefit of fotemustine! Abstract 9502
-median OS 1 year -lack of treatments
-fotemustine- alkylating agent: RR and PFS in 171 liver metastatic was previously used in metastatic melanoma
primary endpoint- MFS (Metastatic Free Survival)
secondary endpoints- OS and quality life
arm A-fotemustine
arm B -monitoring
---------
302 patients enrolled
Patients -genomic High Risk in both of arms
3 years- 60,9% MFS and 59,3%
83, 1 OS at 3 years
Fotemustine fail to improve MFS (metastatic free survival) 3 year (60,9 vs 59,3).
Interimar analysis and trial stopped for futility!